Through their incredibly effective mechanisms of action, cell-based therapies are an emerging technique that hold the promise of treating a wide range of diseases that are now incurable. Cell-based therapies continue to face many obstacles that prevent their widespread translation and commercialisation, despite notable recent clinical and commercial successes. These obstacles include identifying the right cell source, developing sufficiently viable, potent, and safe products that meet patient- and diseasespecific needs, and creating scalable manufacturing processes. Advanced fundamental research, fueled by next-generation engineering techniques, such as genome and epigenome editing, synthetic biology, and the use of biomaterials, is being used to overcome these obstacles.
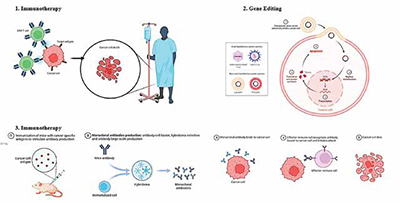
Biological therapy uses the body's immune system to combat cancer, using cells, antibodies, and organs to protect against invaders and differentiate between healthy and cancerous cells, potentially eliminating them. A few treatments have cleared regulatory obstacles and been available for purchase, which has increased public awareness and enthusiasm. Among these is the effective treatment of lymphoid cancers through genetically reprogrammed T cell adoptive cell transfer, which led to the FDA approving tisagenlecleucel and axicabtagene ciloleucel in 2017 for the treatment of large B cell lymphoma (LBCL) and acute lymphoblastic leukaemia (ALL), respectively. In the 1950s, bone marrow transplants were introduced as cell-based therapies for patients with blood-borne cancers. Other notable achievements in recent times include the authorisation of the use of adult stem cells to treat fistulas connected to Crohn's disease and patient-derived limbal stem cells to restore damaged corneal epithelium. These innovations were the result of decades of fundamental study, and their successes—along with those of other cutting-edge treatments—have greatly increased interdisciplinary interest from a wide range of so far disconnected basic biological research and engineering disciplines.
Such continued excitement surrounding cell-based therapeutics stems from the possibility of rerouting natural cellular processes to allow for safety and effectiveness profiles that surpass those of other, more proven techniques. Biologics, which include recombinant proteins and other cell-derived biomolecules, can achieve high target specificity by utilising macromolecules' recognition capabilities, but their safety and efficacy may be limited by unfavourable pharmacokinetic (PK) and pharmacodynamic (PD) properties. With therapeutic transgene delivery, often via a viral vector, gene treatments provide the possibility of rectifying cellular genotype. Cell-based therapies, despite facing translational barriers like tumorigenicity and high manufacturing costs, possess unique intrinsic features such as migration, localisation and proliferation that could enhance efficacy against disease. Despite advancements in cellderived therapies for various purposes, commercialisation remains challenging due to the need for readily available cell sources.
Biological medicines often function by various mechanisms including inducing an immunological response against cancerous cells. Treatments using biological therapy can do this in a number of ways. Introducing substances into the body that stimulate the immune system is one method. Another involves reintroducing a person's immune system cells into their body after they have been trained to combat cancer cells in-vitro. Targeting the cancer cells with biological treatment allows them to switch on or off cell signals that aid in their immune system evasion. Immune checkpoint inhibitors, for instance, are medications that specifically target certain receptors on the surface of cancer cells. There, they obstruct the signals that cancer cells transmit in an effort to hide from the immune system.
The process of adoptive T cell transfer entails isolating and reintroducing T cells into patients in order to cure illnesses. The process's ultimate goal is to stimulate and expand powerful, antigen-specific T cell immunity, which is theoretically identical to the goal of a successful T cell immunisation. One of the major drawbacks of vaccine-based approaches, which is the need to de-novo activate and expand a tumour antigen-specific T cell response in patients who are frequently immunocompromised and highly tolerant to cancer antigens or antigens expressed during chronic infection, may also be addressed through adoptive T cell transfer. More than 50 years ago, adoptive cell transfer with the purpose of targeting illness was initially documented in rodent models. Expanding ex vivo large numbers of T cells for adoptive immunotherapy has been made easier by advances in T cell biology, including mechanisms for T cell activation and target recognition, the function of accessory surface molecules and signal transduction pathways involved in the regulation of T cell function and survival, and the identification and cloning of soluble T cell growth factors.
Treatment for cancer using chimeric antigen receptor (CAR)-T cell therapy is a ground-breaking novel approach. CAR molecules are primarily composed of four components: (i) an intracellular signalling domain that facilitates T cell activation (CD3 ); (ii) a hinge region; (iii) a transmembrane domain; and (iv) an extracellular target antigen-binding domain, which is often an antibodyderived single-chain variable fragment (scFv). Third-generation CARs have two costimulatory domains, whereas second-generation CARs only contain one co-stimulatory intracellular domain (often CD28 or 4-1BB). The production of secreting molecules (cytokines, T cell engagers, agonists or inhibitors of various cell receptors, etc.) or membrane receptors (such as chemokine receptors) is another characteristic of fourth generation CAR-T cells that modifies their efficiency. Currently, relapsed or refractory (r/r) haematological malignancies are the only conditions for which CAR-T cell treatments have been given FDA and EMA approval for commercialisation. Tisagenlecleucel (KymriahTM), the first CAR-T treatment, was permitted by the FDA in 2017 to treat r/r acute lymphoblastic leukaemia (ALL), and in 2018 it was also licenced to treat r/r diffuse large B cell lymphoma (DLBCL). Lisocabtagene maraleucel (BreyanziTM) was approved in 2021 for r/r large B cell lymphoma, primary mediastinal large B cell lymphoma, DLBCL, and grade 3B follicular lymphoma. The same year, idecabtagene vicleucel (AbecmaTM) for r/r multiple myeloma was commercialised. Ciltacabtagene autoleucel (CarvyktiTM) was the latest CAR-T cell treatment to get FDA and EMA approval in 2022 for multiple myeloma. Furthermore, in 2021, the Spanish Agency of Medicines and Medical Devices (AEMPS) authorised for ALL, under a unique local authorisation known as a "hospital exemption," the product ARI-0001, a CAR-T cell therapy developed at the Hospital Clínic of Barcelona that is not industrially manufactured. This is the first medicine of its kind to be approved in all of Europe. The therapeutic efficiency of CAR-T cells in solid tumours and haematological malignancies is limited by several obstacles, despite the fact that therapy with CAR-T cells has achieved significant clinical responses with some subsets of B cell leukaemia or lymphoma. Severe, potentially fatal toxicities, weak antitumor activity, antigen evasion, restricted trafficking, and restricted tumour invasion are some of the obstacles to successful CAR-T cell treatment. Furthermore, interactions between CAR-T cells and their host and tumour microenvironment significantly modify CAR-T cell activity. To address these major obstacles, new methods and techniques for creating more potent CAR-T cells with enhanced anti-tumor activity and reduced toxicity are required.
Because of their critical functions in immunity, cytokines are appealing as treatments for a range of immune-related conditions. However, the short blood half-lives and serious side effects brought on by low specificity and unfavourable biodistribution of cytokines have prevented their broad clinical usage. Bioengineering advances have produced new technology for cytokine engineering as well as advances in our understanding of cytokine biology. During immunological reactions, information is exchanged between cells directly or by the release of biomolecules, the most significant of which are tiny proteins called cytokines. Based on their roles, many primary cytokine classes can be differentiated. Pro-inflammatory cytokines, which include tumour necrosis factor (TNF), IL-1 , IL-6, IL-17, and IL-22, activate antimicrobial and immunostimulatory processes. Anti-inflammatory cytokines, such transforming growth factor- (TGF ) and IL-1 receptor antagonist (IL-1RA), reduce inflammation and accelerate wound healing. Another type of cytokines that control immune cell movement are chemokines, which include IL-8 and CC-chemokine ligand 2 (CCL2). Antiviral immunity relies on interferons, and homeostasis and the proliferation of immune cell progenitor cells are regulated by colonystimulating factors such as granulocyte– macrophage colony-stimulating factor (GM-CSF), granulocyte colony-stimulating factor (G-CSF), and macrophage colony-stimulating factor (M-CSF). The majority of cytokines are produced by immune cells, however non-immune cells and tissues can also release cytokines. Improvements in bioengineering have led to new understandings of cytokine biology, including information on the structure, function, and binding processes of receptors as well as cytokine sequence and structure. When cytokine roduction is increased, as in autoimmuneand inflammatory illnesses, blocking cytokine function with monoclonal antibodies or receptor blockers is very effective. Treatment for Crohn's illness and rheumatoid arthritis that works well is blocking TNF31. Suppressing IL-17or IL-23 can be used to treat psoriasis. COVID-19 can be treated with IL-6 and IL-1 antagonists. It is also possible to use cytokines therapeutically to control immunological reactions. Nevertheless, creating medicines based on cytokines is still difficult. The limited therapeutic range of cytokines is attributed to their short blood half-lives, pleiotropism, and poor tissue distribution.
Many promising therapies are available through the developing science of cancer gene therapy. In order to assist effect a cure, a variety of treatment modalities that all involve genetic material to change cells (either in vitro or in vivo) are collectively referred to as gene therapy. Extensive in vitro and preclinical animal models have demonstrated outstanding success in assessing a broad range of gene therapy drugs. For instance, using gene thrapy to produce cancer vaccines, target viruses to cancer cells for lysis and death, cut off the blood supply to the tumour, and introduce genes that either kill the cancer cells or return them to their normal cellular phenotype has been shown to improve survival in lung cancer models. Preclinical gene therapy investigations have been conducted on several malignancies, including gliomas, pancreatic cancer, liver cancer, and many more. There are significant safety concerns,as there are with any novel form of therapy. The death of a patient in a 1999 dose-escalation gene therapy experiment put an end to the initial fervour surrounding gene therapy as a therapeutic approach. All gene therapy studies were reevaluated for safety, even though this one involved using gene therapy to treat ornithine transcarbamylase deficiency, a metabolic disorder, rather for cancer. Thirteen Since then, hundreds of cancer patients worldwide have taken part in gene therapy studies with very few treatment side effects, and safer and newer gene therapy delivery methods have been developed.
The effective introduction of chimeric and genetically altered human immunoglobulin proteins has made monoclonal antibodies (mAbs) a proven treatment option for cancer. The distinct characteristics of monoclonal antibodies (mAbs) such as their elevated affinity and specificity, coupled with the differential expression of target antigen in tumour cells as opposed to normal cells, render them appealing tools for cancer immunotherapy. The goal of the field of immunoconjugate development is to bring together the best features of these two distinct modalities—the cytotoxic and radionuclide compounds, and the selectivity of monoclonal antibody therapy. Gemtuzumab ozogamicin, a drug compound, and 90Y-ibritumomab tiuxetan, a radiolabeled monoclonal antibody, have been authorised for the treatment of cancers. Preclinical and clinical trials are being conducted on other conjugates that carry toxic payloads of peptide exotoxins, maytansinoids, geldanamycin, and calicheamicin.
A new class of cancer therapeutics called oncolytic viruses (OVs) has several advantages over existing ones, including the ability to deliver multiple eukaryotic transgene payloads, induce immunogenic cell death, promote antitumor immunity, and have a tolerable safety profile that largely does not overlap with other cancer therapeutics. It is possible to equip modified OVs with desired foreign genes that may use several methods to produce significant anticancer effects. Though OVs have similar anticancer processes, distinct virus types or subtypes are being investigated for their ability to treat diverse clinical diseases. Depending on the kind of nucleic acid, OVs can be single- or double-stranded DNA or RNA viruses. The first phase is the transformation of wild-type viruses into tumourspecific Ovs. This can occur during infection or replication. It is necessary to conduct the procedure in accordance with the characteristics of the viruses and tumour cells. While genetically modified oncoviruses (OVs) are developed for improved targeting selectivity, various virus types exhibit varying natural affinities and preferred replication tendencies in distinct tumour cells.
Though talimogene laherparepvec (T-VEC) is still the sole generally authorised medication, four oncolytic viruses and one non-oncolytic virus have received approval for the treatment of cancer worldwide so far. T-VEC was first licenced in 2015 and is designed for the treatment of individuals with recurrent melanoma following first surgery. Data from clinical studies of many different drugs are also becoming accessible, along with an increasing amount of information on the clinical experience of patients receiving T-VEC.
As chemotherapy's efficacy and toxicity to normal tissues reach their limitations, researchers are searching for novel treatment plans based on combination and personalised therapy, which will likely shape medical practice in the future. Modern molecular biology techniques, like the NGS approach, assure the evolution of cancer therapeutics by providing a deeper understanding of the biology of cancer and identifying a suitable therapeutic target. This advancement is also made feasible by the application of more sophisticated bioinformatics techniques, which allow for exact medication modification to the therapeutic target.
It is reasonable to predict that biological therapy in general will become more crucial in the management of neoplasms. We provide many strategies for enhancing the bioavailability, binding strength, or stability of CAR T cell treatment, anticancer vaccinations, and antibody structures in the evaluation of a few research. Furthermore, research possibilities that might enhance the efficacy of biological treatment were highlighted. It has been shown that there are advantages to combining different biological therapies, such as immune checkpoint inhibitors with oncolytic viruses and anti-cancer vaccinations.